Protocol for ChromaLIVE™ Deep Red Non-Toxic Dye
This is intended as a guide only.
In Brief Download the PDF of this protocol
ChromaLIVE™ Deep Red Non-Toxic Dye is a ready-to-use fluorescent probe for long term high content phenotypic screening of live cells. Optimised for live cell painting and morphological profiling in 2D monolayers, 3D spheroids or organoids, the dye provides multi organelle read out for unbiased classification of cell states such as proliferation, stress responses, autophagy, apoptosis or other phenotypic transitions. ChromaLIVE™ Deep Red Non-Toxic Dye can be used with NucleoLIVE™ Non-Toxic Dye (Cat. No. 8935) to get even more insight from live cell painting assays.
ChromaLIVE™ is a trademark of Saguaro Biosciences.
1. Protocol Overview
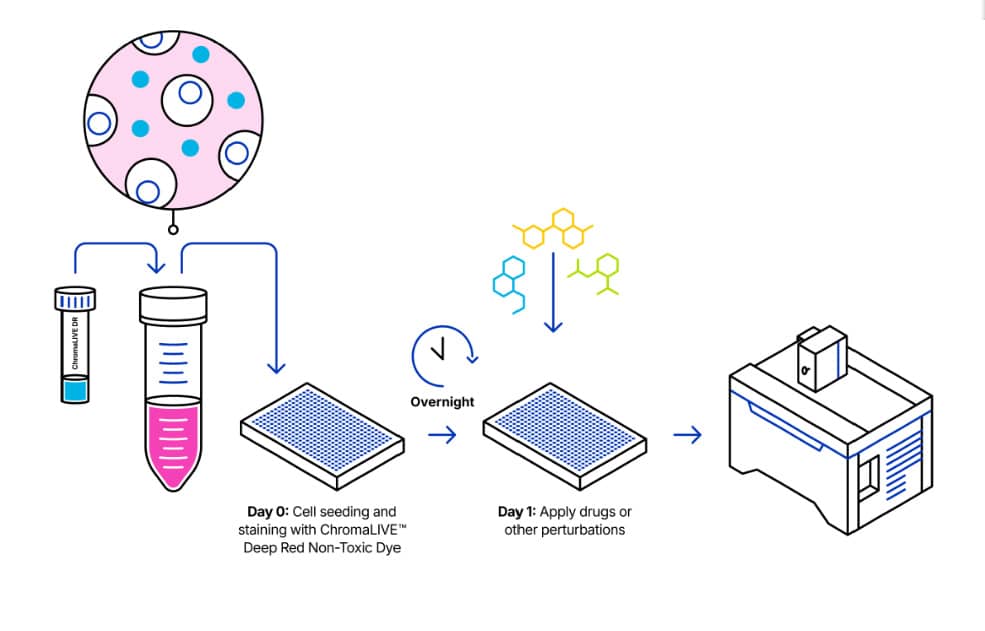
2. Content and Storage
| Product | Content | Storage | Stability |
|---|---|---|---|
|
ChromaLIVE™ Deep Red Non-Toxic Dye |
Diluted in 10 µl of DMSO |
-20º C Delivered at room temperature Protect from light |
1 year |
Table 1. ChromaLIVE™ Deep Red Product Information
Intended Use: For research use only. Not for use in diagnostics or therapeutic procedures.
3. General Guidelines
ChromaLIVE™ Deep Red Dye Dilution and Preparation
- Warm up the ChromaLIVE™ Deep Red Dye tube to room temperature before use to avoid condensation to form and water to get into the anhydrous dye solution
- Gently spin the tube before use to collect any dye solution that may remain near the cap
- Dilute ChromaLIVE Deep Red Dye 1,000-fold in preferred cell culture medium
- Vortex thoroughly
- Seed cells at desired density (typically to achieve 70-80% confluence) in cell culture medium containing ChromaLIVE™ Deep Red Dye in a black multi-well plate. Return to the incubator at 37°C, 5% CO2 overnight
- No washing step is required prior to imaging. Keep ChromaLIVE™ Deep Red in solution throughout the assay
Alternative Cell Culture Indications for ChromaLIVE™ Deep Red
- While we recommend seeding cells in the presence of diluted ChromaLIVE™ Deep Red, the dye can be added after cell seeding, before or following compound addition. Optimisation of seeding density and incubation times prior to imaging are required. For reference, ChromaLIVE™ Deep Red staining stabilizes after 12 hours in U2OS cells
- A nuclear dye can be added to allow cell segmentation during data analysis, such as the NucleoLIVE™ Non-Toxic Dye (Cat. No. 8935) . We recommend running a preliminary imaging test on cells treated with single dyes to validate the staining kinetics and absence of fluorescence bleed-through between the nuclear dye and ChromaLIVE™ Deep Red channels on your system
Imaging Parameters
- Two wavelengths (Recommended): ChromaLIVE™ Deep Red dye needs to be imaged at two different wavelengths minimally: ChromaLIVE640, and either ChromaLIVE488_Yellow or ChromaLIVE488_Red. Selecting only one of the two CL488 channels is sufficient for differentiating between cellular phenotypes, even subtle ones
- Three wavelengths (Optional): While ChromaLIVE488_Yellow and ChromaLIVE488_Red look mostly similar (see Figure 2), they can still provide slightly different information. When feasible, acquiring both ChromaLIVE488 channels is recommended to maximize data richness. However, this approach comes with increased acquisition time and larger file sizes, which should be taken into consideration
4. Technical Specifications & Instrument compatibility
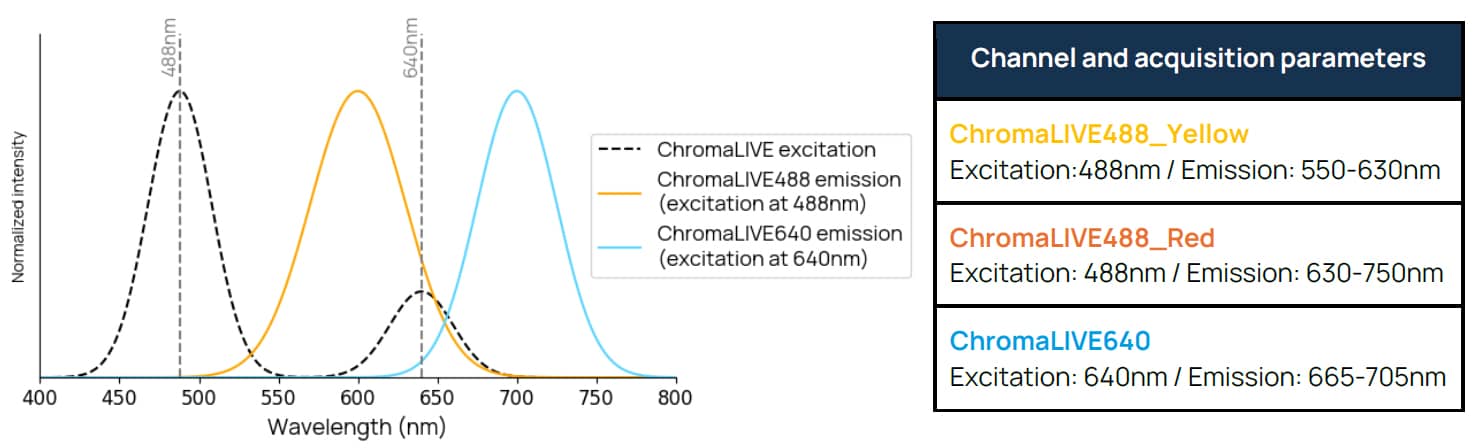
Figure 1. Excitation and emission spectra of ChromaLIVE™ Deep Red. ChromaLIVE™ is excited at 488nm and 640nm, with different resulting emission spectra. In orange, ChromaLIVE488 emission when excited around 488nm. In cyan, ChromaLIVE640 emission spectrum when excited around 640nm.
Instrument compatibility
| Manufacturer | Instrument | Settings | Filters | Mode |
|---|---|---|---|---|
| Molecular Devices | ImageXpress Confocal | ChromaLIVE488_Yellow | Cyan / FITC / Cy3 | Widefield / Confocal |
| ImageXpress Confocal HT.ai | ChromaLIVE488_Red | Cyan / FITC / Cy5 | Widefield / Confocal | |
| ChromaLIVE640 | Red / Cy5 / Cy5 | Widefield / Confocal | ||
| PerkinElmer / Revvity | Opera Phenix | ChromaLIVE488_Yellow | 488 / 570-630 | Widefield only |
| Opera Phenix Plus | ChromaLIVE488_Red | 488 / 650-760 | Widefield / Confocal | |
| ChromaLIVE640 | 640 / 650-760 | Widefield / Confocal | ||
| Operetta CLS | ChromaLIVE488_Yellow | 460-490 / 570-650 | Widefield only | |
| ChromaLIVE488_Red | 460-490 / 655-760 | Widefield / Confocal | ||
| ChromaLIVE640 | 615-645 / 655-760 | Widefield / Confocal | ||
| Yokogawa | CQ1 | ChromaLIVE488_Yellow | 488 / 617/73 | Confocal |
| ChromaLIVE488_Red | 488 / 685/40 | Confocal | ||
| ChromaLIVE640 | 640 / 685/40 | Confocal | ||
| CV8000 | ChromaLIVE488_Yellow | 488 / 600/37 | Confocal | |
| ChromaLIVE488_Red | 488 / 676/29 | Confocal | ||
| ChromaLIVE640 | 640 / 676/29 | Confocal |
* ChromaLIVE™ Deep Red is compatible with other high-content imagers and confocal microscopes. Please refer to Figure 1 for technical specifications.
4.1 Image Examples and Recommended Positive Control Compounds*
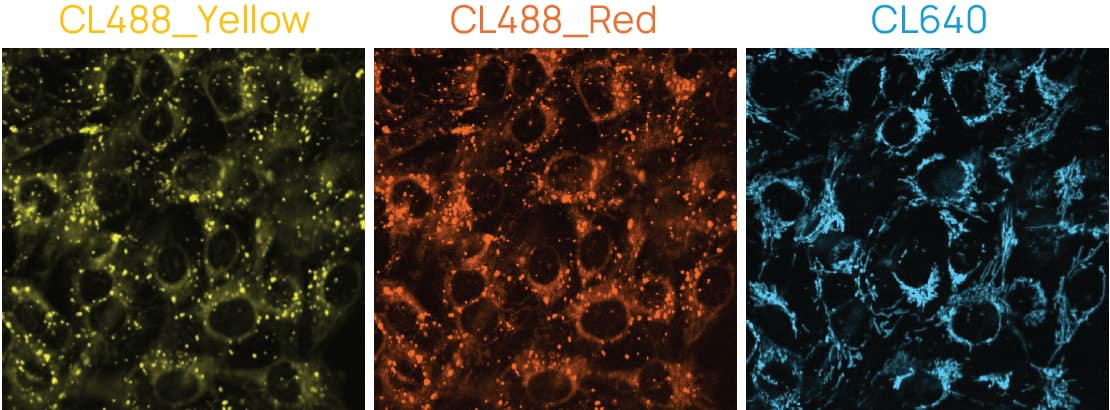
Figure 2. MCF-7 cells stained with ChromaLIVE™ Deep Red. Yellow: ChromaLIVE488_Yellow, Orange: ChromaLIVE488_Red, Cyan: ChromaLIVE640.
Table 2. Doses and treatment durations for MCF7 cells in 2D. Bold represents recommended assay endpoint
| Cell Death Mechanism | Apoptosis | ER Stress | Autophagy |
|---|---|---|---|
|
Control Compound |
Actinomycin D (1 pM-1 μM)
|
Tunicamycin (10 pM-10 μM)
|
Rapamycin (10 pM-10 μM) 12h, 24h, 48h, 72h |
DMSO Early Apoptotic Apoptotic ER Stress Autophagy
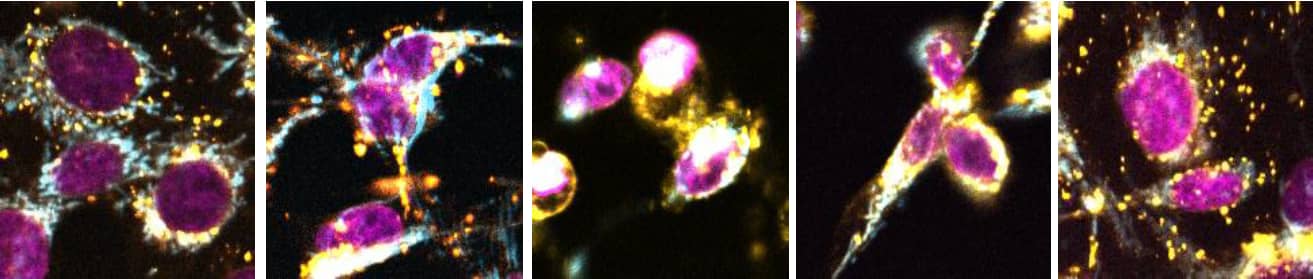
Figure 3. MCF-7 cells with ChromaLIVE™ Deep Red and NucleoLIVE™. Cells were treated for an early apoptotic or apoptotic phenotype (staurosporine 5 nM and 500 nM for 24h, respectively), an ER stress (thapsigargin 1 nM, 24h) or an autophagy phenotype (rapamycin 100 nM, 72h). Magenta: NucleoLIVE™, Yellow:CL488_Yellow, Orange: CL488_Red, Cyan: CL640.
* Compounds provided as examples only. Validation required for each experimental protocol.
** Images could be collected more frequently with the appropriate equipment, especially for time-lapse imaging (controlled temperature and CO2, auto-focusing, etc.)
4.2. Example Protocol (for kinetic, 2D live-cell assay)
MCF7 cells treated with standard compounds for apoptosis, ER stress and autophagy. MCF7 are cultured in RPMI 1640 complemented with 10% FBS and 1% Penicillin/Streptomycin.
ChromaLIVE™ Deep Red and NucleoLIVE™ Dye Dilution and Preparation (Day 0):
• Warm up the ChromaLIVE™ and NucleoLIVE™ dye tubes to room temperature before use and gently spin to collect any dye solution that may remain near the cap
• Dilute 10 μL ChromaLIVE™ Deep Red dye in 10 mL culture medium (1000-fold)
• Dilute 10 μL NucleoLIVE™ dye in the same 10 mL culture medium (1000-fold)
• Vortex thoroughly
Cell Culture Protocol with ChromaLIVE™ Deep Red (Day 0):
• Harvest and count MCF7 cells
• Resuspend cells in prepared culture medium with ChromaLIVE™ Deep Red and NucleoLIVE™ dyes at 80,000 cells/mL
• Seed 96-well plate with 100μL cell suspension per well to a final density of 8,000 cells per well
• Incubate overnight at 37°C, 5% CO2
Standard Compound Preparation and Addition (Day 1):
• Prepare dose-response curves with 10x concentrations, maintaining constant vehicle (0.1% DMSO) solvent concentration
• Prepare negative controls with 0.1% DMSO in complete media
• Distribute 12.5 μL of test compounds or controls per well
Imaging and Data Acquisition (Days 1-3):
• Image 96-well plate at 3h, 6h, 24h and 48h after addition of test compounds