BromoCatch™ Ligands Usage Protocols
This is intended as a guide only; for full experimental details please read the reference provided.
In Brief Download the PDF of this protocol
BromoCatch™ is a covalent protein tagging system based on engineered bromodomains that selectively and rapidly react with an electrophilic ligand. This enables irreversible labeling of BromoCatch™ fusion proteins in live cells, fixed cells, or lysates.
BromoCatch™ Ligands include:
- Biotin BromoCatch™ Ligand (Cat. No. 8939)
- BromoCatch™ Control Ligand (Cat. No. 7300)
- BromoCatch™ Ligand, Alkyne (Cat. No. 8940)
- Janelia Fluor® 525, BromoCatch™ Ligand (Cat. No. 8997)
- Janelia Fluor® 585, BromoCatch™ Ligand (Cat. No. 8998)
- Janelia Fluor® 635, BromoCatch™ Ligand (Cat. No. 8937)
- Janelia Fluor® 549, BromoCatch™ Ligand (Cat. No. 8942)- Coming soon!
- TAMRA, BromoCatch™ Ligand (Cat. No. 8938)- Coming soon!
Reagent Handling & Reconstitution
Recommended reconstitution details:
| Reagent | Catalog No. | Supplied Amount | Stock Concentration | DMSO Reconstitution Volume |
|---|---|---|---|---|
| Biotin BromoCatch™ Ligand | 8939 | 50 µg | 1 mM | 55 µL |
|
BromoCatch™ Control Ligand |
7300 | 100 µg | 1 mM | 226 µL |
| BromoCatch™ Ligand, Alkyne | 8940 | 50 µg | 1 mM | 79 µL |
|
Janelia Fluor® 525, BromoCatch™ Ligand |
8997 | 10 µg | 200 µM | 44 µL |
| Janelia Fluor® 585, BromoCatch™ Ligand | 8998 | 10 µg | 200 µM | 43 µL |
| Janelia Fluor® 635, BromoCatch™ Ligand | 8937 | 10 µg | 200 µM | 43 µL |
Tips:
- Spin down contents before opening
- Store reconstituted stock at –20 °C protected from light
- Avoid repeated freeze–thaw cycles
- BromoCatch™ Control Ligand can be used at X concentration to compete with other BromoCatch™ probes in control experiments
Cell lysate labeling Protocol
Example data generated using H2B, generalizable protocol for your protein of interest (POI).
Materials & Reagents
- HEK293-FT cells
- pCMV POI-BromoCatch vector
- DMEM, Optimem, FBS (10%)
- TAMRA, BromoCatch™ Ligand (Cat. No. 8938)
- DMSO (control)
- RIPA buffer
- Primary anti-H2B antibody (Rabbit)
- Secondary antibody (IR800)
- 6 or 12 well plates
- Humidified incubator (5% CO₂, 37°C)
- Trypsin
- Western blot reagents
Protocol
- Transfection: Transfect HEK293-FT cells with the pCMV POI-BromoCatch vector using standard transfection reagents and incubate in a humidified incubator (5% CO₂, 37°C) for at least 16 hours.
- Cell Plating: Trypsinize transfected cells and plate them onto 6 or 12 well plates and allow cells to adhere for 16 hours.
- Treatment with Probe: Replace DMEM with Optimem + 10% FBS, containing either DMSO (control) or increasing concentrations of TAMRA, BromoCatch™ Ligand.
- Incubation: Incubate treated cells for 2 hours in a humidified incubator (5% CO₂, 37°C).
- Cell Washing: Gently wash cells with warm Optimem + 10% FBS media to remove excess probe.
- Cell Lysis: Lyse cells using RIPA buffer to extract proteins.
- Western Blotting & Membrane Transfer: Perform SDS-PAGE and transfer proteins onto a membrane.
- Fluorescence Imaging: Directly image the membrane using the TMR fluorescence channel.
Control: POI Antibody Staining (if required)
9. Primary Antibody Staining: Incubate membrane with anti-H2B antibody.
10. Secondary Antibody Staining: Apply IR800-conjugated secondary antibody for detection.

Figure 1. TAMRA probes for cell lysate experiments.
TAMRA, BromoCatch™ Ligand (Cat. No. 8938) specifically detects H2B-BromoCatch at 0.25 μM concentration of probe. The probe showed no unspecific binding in HEK293FT WT cells when incubated at up to 2.5 μM.
Live-Cell Labeling Protocol (Fluorescent Probes)
Materials & Reagents
- U2-OS cells
- pCMV POI-BromoCatch vector
- DMEM medium
- Optimem + 10% FBS
- Humidified incubator (5% CO₂, 37°C)
- DMSO
- Janelia Fluor® 635, BromoCatch™ Ligand (Cat. No. 8937)
- Hoechst 33342 (Cat. No. 5117)
- Trypsin
- Confocal microscope
Protocol
- Transfection: Transfect U2-OS cells with pCMV POI-BromoCatch vector using a suitable transfection reagent and incubate the cells in a humidified incubator (5% CO₂, 37°C) for at least 16 hours.
- Cell Plating: Trypsinise the transfected cells and plate them onto microscopy slides and allow cells to adhere for 16 hours under standard incubation conditions.
- Treatment with Fluorogenic Probe: Replace DMEM medium with Optimem + 10% FBS, supplemented with DMSO (control) or 200 nM Janelia Fluor® 635, BromoCatch™ Ligand.
- Probe Incubation: Incubate cells with the probe for up to 8 hours in a humidified incubator (5% CO₂, 37°C).
- Cell Washing & Staining: Gently wash cells 3 times with warm Optimem + 10% FBS and add Hoechst 33342 to each well and incubate for 30 minutes.
- Imaging: Perform confocal microscopy imaging to analyze probe fluorescence and nuclear staining.
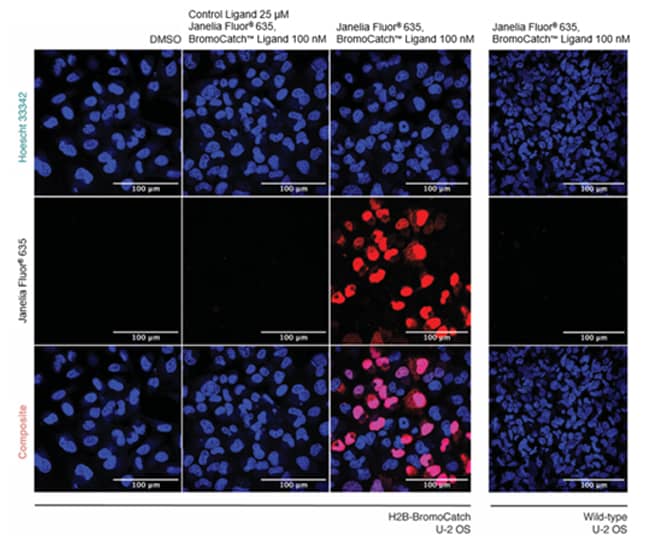
Figure 2: Cellular validation of Janelia Fluor® 635, BromoCatch™ Ligand using live-cell confocal microscopy.
Pulldown Protocol (Biotin BromoCatch™ Ligand)
Materials & Reagents
- HEK293-FT cells
- pCMV H2B-BromoCatch vector
- DMEM, Optimem, FBS (10%)
- Biotin BromoCatch™ Ligand (Cat. No. 8939)
- DMSO (control)
- RIPA buffer
- Primary anti-POI antibody
- Secondary antibody (IR800)
- 6 or 12 well plates
- Humidified incubator (5% CO₂, 37°C)
- Trypsin
- Western blot reagents
Protocol
- Transfection: Transfect HEK293-FT cells with the pCMV H2B-BromoCatch vector using standard transfection reagents and incubate in a humidified incubator (5% CO₂, 37°C) for at least 16 hours.
- Cell Plating: Trypsinize transfected cells and plate them onto 6 or 12 well plates and allow cells to adhere for 16 hours.
- Treatment with probe: Replace DMEM with Optimem + 10% FBS, containing either DMSO (control) or increasing concentrations of Biotin BromoCatch™ Ligand.
- Incubation: Incubate treated cells for 2 hours in a humidified incubator (5% CO₂, 37°C).
- Cell Washing: Gently wash cells with warm Optimem + 10% FBS media to remove excess probe.
- Cell Lysis: Lyse cells using RIPA buffer to extract proteins.
- Western Blotting & Membrane Transfer: Perform SDS-PAGE and transfer proteins onto a membrane.
- Streptavidin TMR antibody incubation: Image membrane using the TMR fluorescence channel.
Control: POI Antibody Staining (if required)
9. Primary Antibody Staining: Incubate membrane with Rabbit anti-H2B antibody.
10. Secondary Staining: Apply IR800-conjugated secondary antibody for detection.

Figure 3: Biotin probes for cell lysate experiments.
Biotin BromoCatch™ Ligand (Cat. No. 8939) specifically detects H2B-BromoCatch at 0.25 μM concentration of probe. The probe showed no unspecific binding in HEK293FT WT cells when incubated at up to 2.5 μM.
Click Chemistry Protocol (Alkyne BromoCatch™ Ligand)
- Label cells or protein with 250 nM–1 µM ligand.
- Perform CuAAC with azide-fluorophore, CuSO4, ligand, and sodium ascorbate.
- Incubate 30 min at RT, then wash thoroughly.
- Proceed to analysis depending on azide used (e.g., fluorescence etc_)
Plasmids
To streamline adoption of the BromoCatch™ platform, we have a comprehensive suite of ready-to-use plasmids for mammalian expression of BromoCatch tagged proteins. These constructs are optimized for flexible cloning and high-level expression in a range of cell types, supporting both N- and C-terminal fusions to your protein of interest.
Each vector includes a BromoCatch domain flanked by flexible glycine-serine linkers (GSL) and multiple cloning sites, enabling modular insertion of target sequences. Options are available with or without N- or C-terminal His-tags for affinity purification, and with your choice of CMV or TK promoters for high or moderate expression, respectively. Vectors are available with puromycin or hygromycin B selection markers to suit diverse experimental workflows.
Whether you're performing live-cell imaging, pull-down assays, or proximity labeling, these plasmids provide a reliable and efficient starting point for generating BromoCatch tagged fusion proteins.
| Catalog Number | Backbone | Insert Design |
|---|---|---|
| RDEH-BC01 | CMV promoter, Puromycin | N-term BromoCatch/GSL/cloning sites |
| RDEH-BC02 | CMV promoter, Puromycin | cloning sites/GSL/C-term BromoCatch |
| RDEH-BC03 | CMV promoter, Puromycin | N-term His/ BromoCatch /GSL/cloning sites |
| RDEH-BC04 | CMV promoter, Puromycin | cloning sites/GSL/C-term BromoCatch /His |
| RDEH-BC05 | CMV promoter, Hygromycin B | N-term BromoCatch /GSL/cloning sites |
| RDEH-BC06 | CMV promoter, Hygromycin B | cloning sites/GSL/C-term BromoCatch |
| RDEH-BC07 | CMV promoter, Hygromycin B | N-term His/BromoCatch/GSL/cloning sites |
| RDEH-BC08 | CMV promoter, Hygromycin B | cloning sites/GSL/C-term BromoCatch/His |
| RDEL-BC01 | TK promoter, Puromycin | N-term BromoCatch/GSL/cloning sites |
| RDEL-BC02 | TK promoter, Puromycin | cloning sites/GSL/C-term BromoCatch |
| RDEL-BC03 | TK promoter, Puromycin | N-term His/BromoCatch/GSL/cloning sites |
| RDEL-BC04 | TK promoter, Puromycin | cloning sites/GSL/C-term BromoCatch/His |
| RDEL-BC05 | TK promoter, Hygromycin B | N-term BromoCatch/GSL/cloning sites |
| RDEL-BC06 | TK promoter, Hygromycin B | cloning sites/GSL/C-term BromoCatch |
| RDEL-BC07 | TK promoter, Hygromycin B | N-term His/BromoCatch/GSL/cloning sites |
| RDEL-BC08 | TK promoter, Hygromycin B | cloning sites/GSL/C-term BromoCatch/His |
BromoCatch™ GFP Expression Plasmids are also available.
BromoCatch™ is a trademark of Bio-Techne Corporation.
Janelia Fluor® is a registered trademark of Howard Hughes Medical Institute.