Protocol for ER-LIVE™ Endoplasmic Reticulum Dye
This is intended as a guide only.
In Brief Download the PDF of this protocol
ER-LIVE™ Endoplasmic Reticulum Dye (Cat. No. 9010) is a ready-to-use fluorescent probe for long term imaging of ER in live cells. ER-LIVE™ Endoplasmic Reticulum Dye can be multiplexed with ChromaLIVE™ Deep Red Non-Toxic Dye (Cat. No. 9009) for phenotypic screening.
1. Protocol Overview
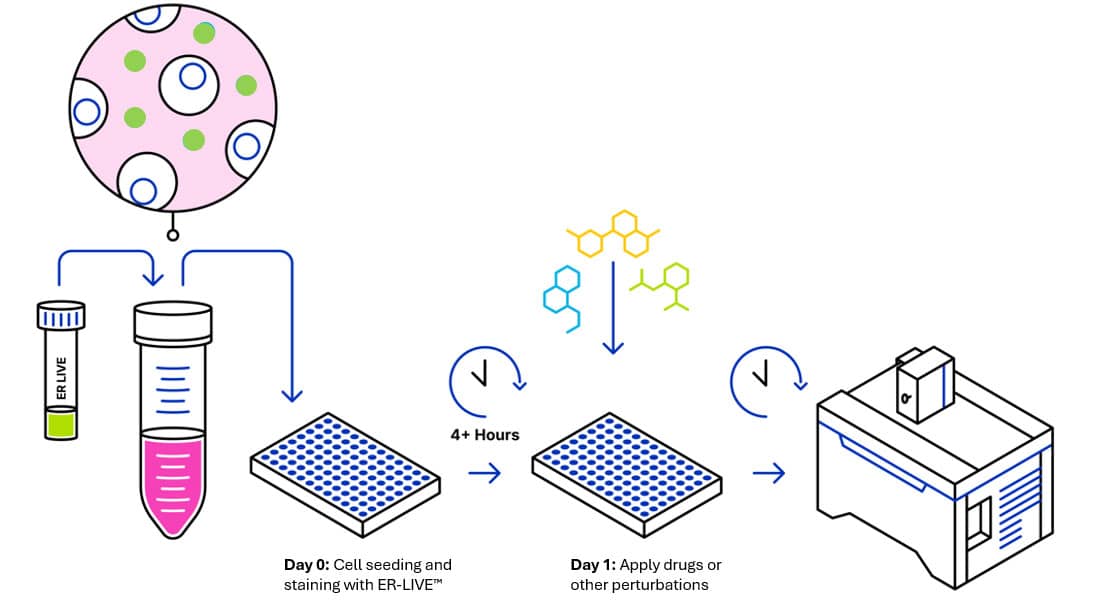
2. Content and Storage
| Product | Content | Storage | Stability |
|---|---|---|---|
| ER-LIVE™ Endoplasmic Reticulum Dye | Diluted in 50 µL of DMSO |
-20º C Delivered at room temperature Protect from light |
1 year |
Table 1. ER-LIVE™ Dye Product Information
Intended Use: For research use only. Not for use in diagnostics or therapeutic procedures.
3. General Guidelines
ER-LIVE™ Dye Dilution and Preparation
- Warm up the ER-LIVE™ tube to room temperature before use to avoid condensation to form and water to get into the anhydrous dye solution
- Gently spin the tube before use to collect any dye solution that may remain near the cap
- Dilute ER-LIVE™ dye 1,000-fold in preferred cell culture medium
- Vortex thoroughly
- Note: We recommend 1X as a starting point for optimization. Higher or lower concentrations may be optimal for different imaging systems and cell models.
- Seed cells at desired density (typically to achieve 70-80% confluence) in cell culture medium containing ER-LIVE™ dye in a black multi-well plate. Return to the incubator at 37°C, 5% CO2 overnight
- No washing step is required prior to imaging. Keep ER-LIVE™ in solution throughout the assay
Alternative Cell Culture Indications for ER-LIVE™ dye
- While we recommend seeding cells in the presence of diluted ER-LIVE™, the dye can be added after cell seeding, before or following compound addition. Optimisation of seeding density and incubation times prior to imaging are required. For reference, ER-LIVE™ staining stabilizes after 4 hours in U2OS cells (at a concentration of 1X)
- A nuclear dye can be added to allow cell segmentation during data analysis, such as NucleoLIVE™ Non-Toxic Dye (Cat. No. 8935). We recommend running a preliminary imaging test on cells treated with single dyes to validate the staining kinetics and absence of fluorescence bleed-through between the nuclear dye and ER-LIVE™ channels on your system.
4. Technical Specifications & Instrument Compatibility
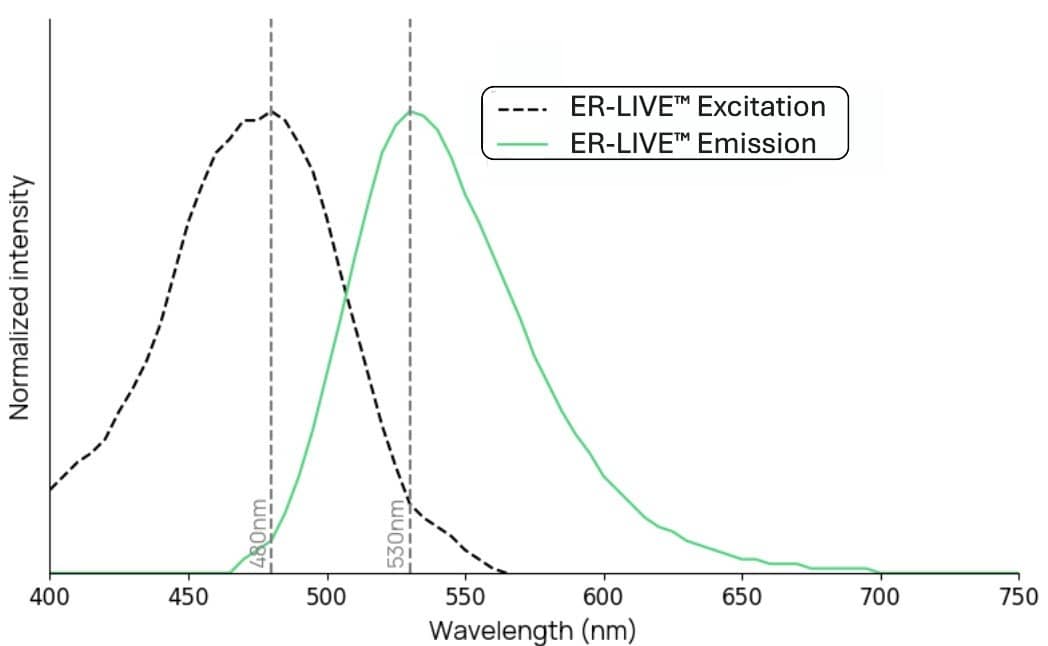
Figure 1. Excitation and emission spectra. ER-LIVE™ is excited at 480nm and emits around 530nm.
| Manufacturer | Instrument | Filters | Mode |
|---|---|---|---|
| Molecular Devices | ImageXpress Confocal | Cyan / FITC / Cy3 | Widefield /Confocal |
| ImageXpress Confocal HT.ai | |||
| PerkinElmer /Revvity | Opera Phenix | 488/ 500-550 | Widefield only |
| Opera Phenix Plus | 460-490 / 500-550 | Widefield /Confocal | |
| Widefield /Confocal | |||
| Operetta CLS | |||
| Yokogawa | CQ1 | 488 / 525-550 | Confocal |
| CV8000 |
Table 2. Instrument compatibility of ER-LIVE™
* ER-LIVE™ is compatible with other high-content imagers and confocal microscopes. Please refer to Figure 1 for technical specifications.
5. ER-LIVE™ Non-toxic Dye, Allowing for High Quality Staining without Compromising Cell Health
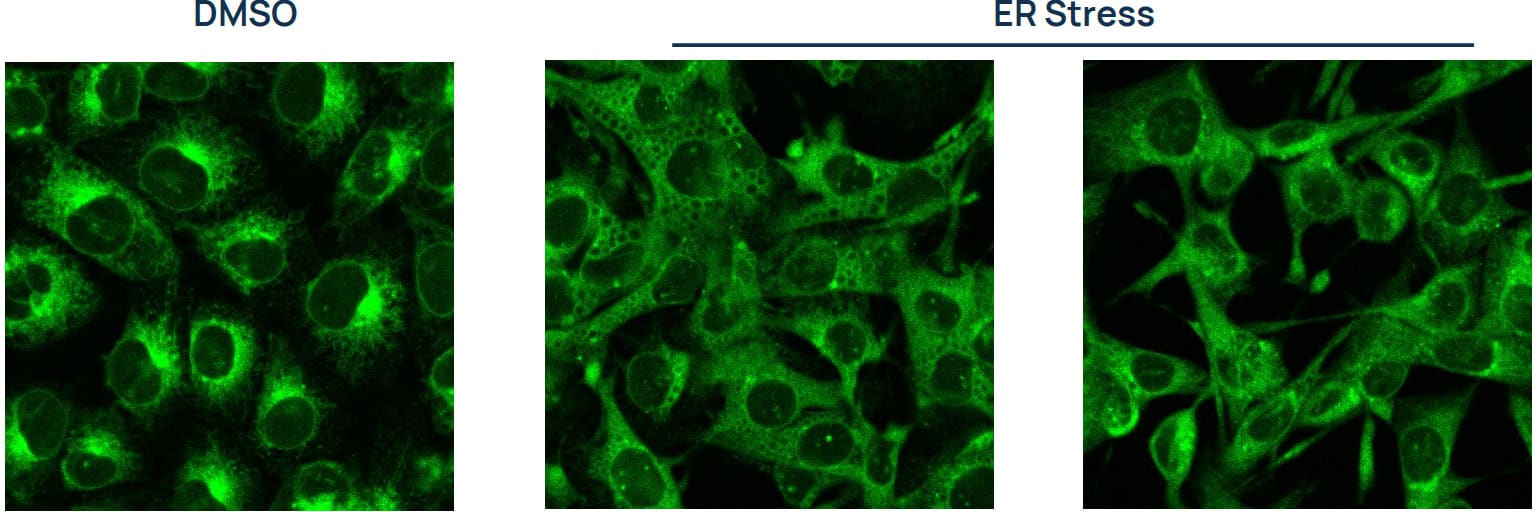
Figure 2. MCF7 cells stained with ER-LIVE™. Composite images of cells undergoing ER stress following Thapsigargin treatment for 24h (middle: 1nM; right: 50nM) compared to an untreated DMSO control. Recommended positive control compounds and treatment durations to induce ER stress (for MCF7 cells). Bold represents recommended assay endpoint.
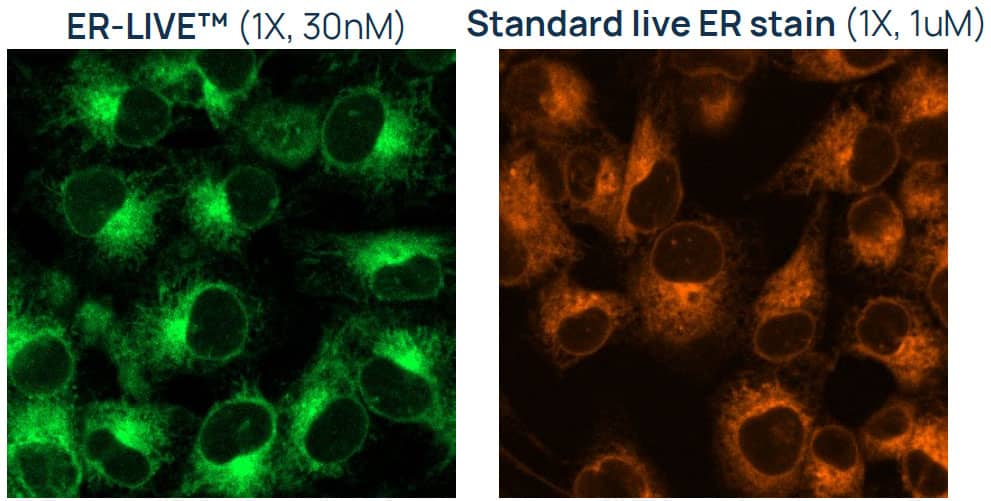
Figure 3. ER-LIVE™ delivers bright, stable endoplasmic reticulum labeling at nanomolar concentrations, minimizing disruption of native cell biology. MCF7 cells stained with ER-LIVE™ (green) or a standard live ER stain (orange) for 24 hours. ER-LIVE™ provides comparable brightness at much lower concentrations, and its mix-and-read formulation allows continuous use in culture for extended imaging or kinetic studies without perturbing cell physiology.
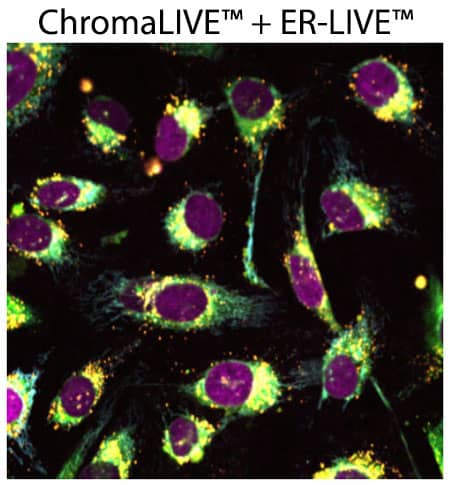
Figure 4. ER-LIVE™ can be multiplexed with ChromaLIVE™ and NucleoLIVE™ to gain deeper biological insights. U2OS cells stained with ChromaLIVE™ (Orange: CL488_Red, Cyan: CL640), ER-LIVE™ (green) and NucleoLIVE™ Red (magenta, CL561).
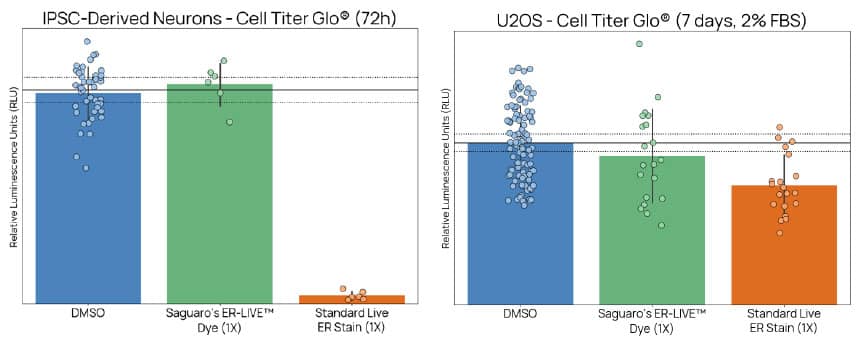
Figure 5. ER-LIVE™ preserves normal cell proliferation and viability, even in sensitive models. iPSC-derived neurons and U2OS cells (maintained in 2% FBS for increased sensitivity) showed comparable viability to DMSO controls after 24-hour incubation with ER-LIVE™. In contrast, the standard live ER dye significantly reduced cell health - demonstrating ER-LIVE™’s non-toxic, non-disruptive profile, enabling extended live-cell imaging without compromising cellular physiology. Both dyes were used at their respective recommended 1X concentrations.
6. Recommended Positive Control Compounds for ER Stress*
| Tunicamycin | Thapsigargin | |
|---|---|---|
|
Control Compound |
10 pM-10 μM |
1 pM-1 μM 3h, 6h, 12h, 24h |
Table 3. Doses and treatment durations for MCF7 cells in 2D. Bold represents recommended assay endpoint.
* Compounds provided as examples only. Validation required for each experimental protocol and cell model.
** Images could be collected more frequently with the appropriate equipment, especially for time-lapse imaging (controlled temperature and CO2, auto-focusing, etc.)
7. Example Protocol (for kinetic, 2D live-cell assay)
MCF7 cells treated with standard compounds for ER stress and apoptosis. MCF7 are cultured in RPMI 1640 complemented with 10% FBS and 1% Penicillin/Streptomycin.
ER-LIVE™ Dye Dilution and Preparation (Day 0):
• Warm up the ER-LIVE™ dye tube to room temperature before use and gently spin to collect any dye solution that may remain near the cap
• Dilute 10 μL ChromaLIVE™ Deep Red dye in 10 mL culture medium (1000-fold)
• Optional: Dilute 10 μL NucleoLIVE™ dye in the same 10 mL culture medium (1000- fold)
• Vortex thoroughly
Cell Culture Protocol with ER-LIVE™ (Day 0):
• Harvest and count MCF7 cells
• Resuspend cells in prepared culture medium with ER-LIVE™ (and NucleoLIVE™, if applicable) at 80,000 cells/mL
• Seed 96-well plate with 100 μL cell suspension per well to a final density of 8,000 cells per well
• Incubate overnight at 37°C, 5% CO2
Standard Compound Preparation and Addition (Day 1):
• Prepare dose-response curves with 10x concentrations, maintaining constant vehicle (0.1% DMSO) solvent concentration
• Prepare negative controls with 0.1% DMSO in complete media
• Distribute 12.5 μL of test compounds or controls per well
Imaging and Data Acquisition (Days 1-3):
• Image 96-well plate at 3h, 6h and 24h after addition of test compounds
ChromaLIVE™, ER-LIVE™ and NucleoLIVE™ are trademarks of Saguaro Biosciences.