Protocol for ChromaLIVE™ Deep Red + ER-LIVE™ Dyes
This is intended as a guide only; for full experimental details please read the reference provided.
In Brief Download the PDF of this protocol
ChromaLIVE™ Deep Red Non-Toxic Dye (Cat. No. 9009) is a ready-to-use fluorescent probe for long-term high-content phenotypic screening of live cells. Optimized for live cell painting and morphological profiling in 2D monolayers, 3D spheroids or organoids, the dye provides multi organelle read out for unbiased classification of cell states such as proliferation, stress responses, autophagy, apoptosis or other phenotypic transitions. ChromaLIVE™ Deep Red Non-Toxic Dye can be used with NucleoLIVE™ Non-Toxic Dye (Cat. No. 8935) to get even more insight from live cell painting assays.
ER-LIVE™ Endoplasmic Reticulum Dye (Cat. No. 9010) is a ready-to-use fluorescent probe for long-term imaging of ER in live cells. ER-LIVE™ Endoplasmic Reticulum Dye can be multiplexed with ChromaLIVE Deep Red Non-Toxic Dye (Cat. No. 9009) for phenotypic screening.
ChromaLIVE™ and ER-LIVE™ are trademarks of Saguaro Biosciences.
1. Protocol Overview
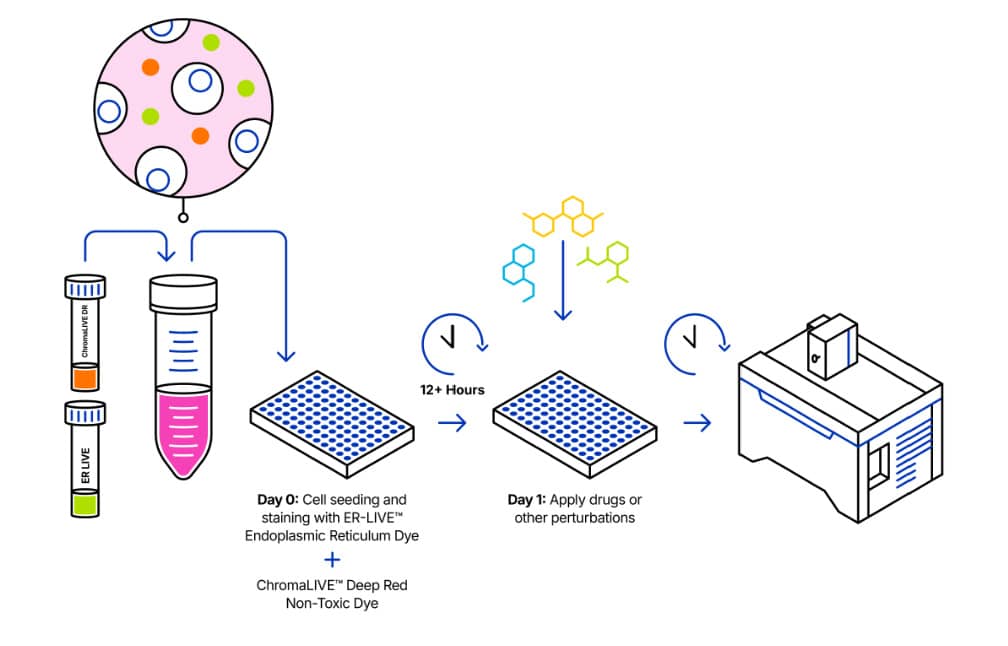
2. Content and Storage
| Product | Content | Storage | Stability |
|---|---|---|---|
| ChromaLIVE™ Deep Red Non-Toxic Dye | Diluted in 10 µL of DMSO |
-20º C Delivered at room temperature Protect from light |
1 year |
| ER-LIVE™ Endoplasmic Reticulum Dye | Diluted in 50 µL of DMSO |
Table 1. ChromaLIVE™ + ER-LIVE™ Dyes Product Information
Intended Use: For research use only. Not for use in diagnostics or therapeutic procedures.
3. General Guidelines
ChromaLIVE™ + ER-LIVE™ Dyes Dilution and Preparation
- Warm up the ChromaLIVE™ and ER-LIVE™ tubes to room temperature to avoid condensation to form and water to get into the anhydrous dye solution
- Gently spin the tubes before use to collect any dye solution that may remain near the cap
- Dilute ChromaLIVE™ and ER-LIVE™ 1,000-fold each in preferred cell culture medium. For 10 mL of media, add 10 µL of ChromaLIVE™ and 10 µL of ER-LIVE™
- Vortex thoroughly
- A nuclear dye can be added to allow cell segmentation during data analysis, such as NucleoLIVE™ Non-Toxic Dye (Cat. No. 8935). We recommend running a preliminary imaging test on cells treated with single dyes to validate the staining kinetics and absence of fluorescence bleed-through between the nuclear dye and ChromaLIVE™ + ER-LIVE™ channels on your system.
- Note: We recommend 1X as a starting point for optimization. Higher or lower concentrations may be optimal for different imaging systems and cell models.
- Seed cells at desired density (typically to achieve 70-80% confluence) in cell culture medium containing ChromaLIVE™ + ER-LIVE™ mix in a black multi-well plate. Return to the incubator at 37°C, 5% CO2 overnight
- No washing step is required prior to imaging. Keep ChromaLIVE™ + ER-LIVE™ mix in solution throughout the assay
Alternative Cell Culture Indications for ChromaLIVE™ + ER-LIVE™
- While we recommend seeding cells in the presence of diluted ChromaLIVE™ + ER-LIVE™, the mix can be added after cell seeding, before or following compound addition. Optimisation of seeding density and incubation times prior to imaging are required. For reference, ChromaLIVE™ + ER-LIVE™ staining stabilizes after 12 hours in U2OS cells
Imaging Parameters for ER-LIVE™
- Three wavelengths (Recommended): ChromaLIVE™ + ER-LIVE™ dyes need to be imaged at three different wavelengths minimally: ChromaLIVE488_Green, ChromaLIVE640, and ChromaLIVE488_Red
- Four wavelengths (Optional): While ChromaLIVE488_Yellow and ChromaLIVE488_Red look mostly similar (see Figure 2), they can still provide slightly different information. When feasible, acquiring both ChromaLIVE488_Red and Yellow channels is recommended to maximize data richness. However, this approach comes with increased acquisition time and larger file sizes, which should be taken into consideration
4. Technical Specifications & Instrument Compatibility
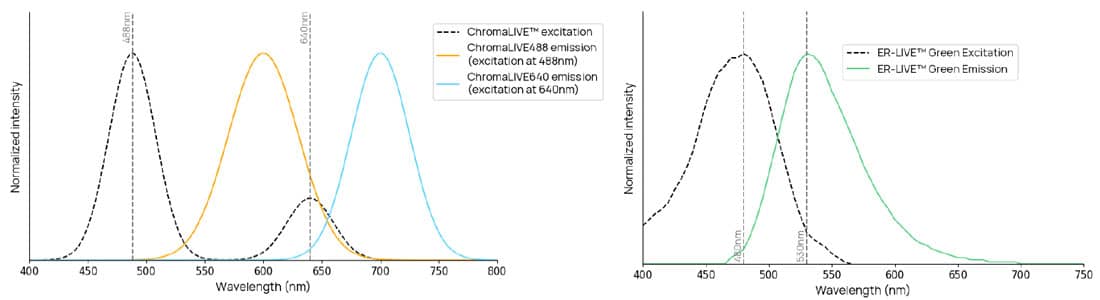
Figure 1. Excitation and emission spectra. ChromaLIVE™ is excited at 488nm and 640nm, with different resulting emission
spectra. In orange, ChromaLIVE488 emission when excited around 488nm. In cyan, ChromaLIVE640 emission spectrum when
excited around 640nm. ER-LIVE™ is excited at 488nm and emits around 530nm.
Table 2. Channels and general acquisition parameters
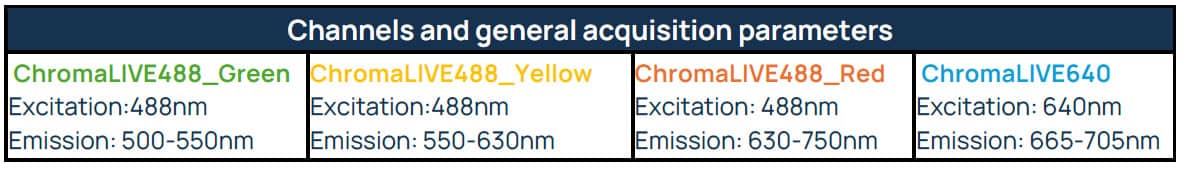
Table 3. Instrument compatibility*
| Manufacturer | Instrument | Settings | Filters | Mode |
|---|---|---|---|---|
| PerkinElmer /Revvity | Opera Phenix | ER-LIVE | 488/ 500 | Widefield / Confocal |
| Opera Phenix Plus | ChromaLIVE488_Yellow | 488 / 570-630 | ||
| ChromaLIVE488_Red | 488 / 650-760 | |||
| ChromaLIVE640 | 640 / 650-760 | |||
| Operetta CLS | ER-LIVE | 460-490 / 500-550 | ||
| ChromaLIVE488_Yellow | 460-490 / 570-650 | |||
| ChromaLIVE488_Red | 460-490 / 655-760 | |||
| ChromaLIVE640 | 615-645 / 655-760 | |||
| Yokogawa | CQ1 | ER-LIVE | 488 / 525-550 | Confocal |
| ChromaLIVE488_Yellow | 488 / 617-673 | |||
| ChromaLIVE488_Red | 488 / 685-640 | |||
| ChromaLIVE640 | 640 / 685-640 | |||
| CV8000 | ER-LIVE | 488 / 525-550 | ||
| ChromaLIVE488_Yellow | 488 / 600-637 | |||
| ChromaLIVE488_Red | 488 / 676/29 | |||
| ChromaLIVE640 | 640 / 676/29 | |||
| Molecular Devices | ImageXpress Confocal | ChromaLIVE488_Yellow + ER-LIVE** | Cyan / FITC / Cy3 | Widefield /Confocal |
| ImageXpress Confocal HT.ai | ChromaLIVE488_Red | Cyan / FITC / Cy5 | ||
| ChromaLIVE640 | Red / Cy5 / Cy5 |
* ChromaLIVE™ Deep Red is compatible with other high-content imagers and confocal microscopes. Please refer to Figure.1 for
technical specifications.
** On some instruments, the ChromaLIVE488_Yellow and ChromaLIVE488_Green (ER-LIVE) channels will be acquired in the
same channel.
5. Image Examples and Performance Comparison to ChromaLIVE™ & Hoechst
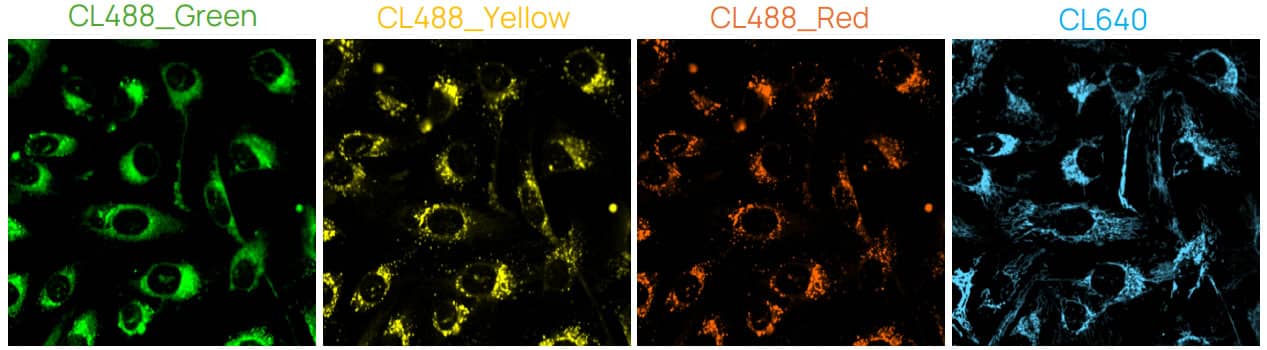
Figure 2. U2OS cells stained with ChromaLIVE™ + ER-LIVE™. Green: ChromaLIVE488_Green, Yellow: ChromaLIVE488_Yellow, Orange: ChromaLIVE488_Red, Cyan: ChromaLIVE640.
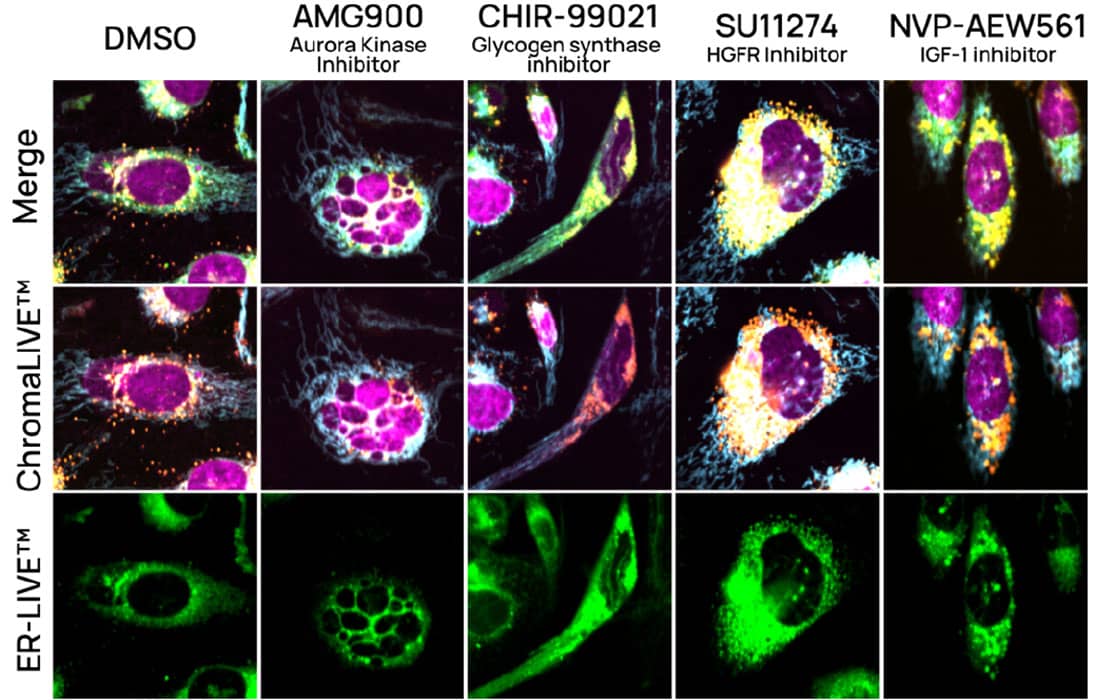
Figure 3. U2OS cells with ChromaLIVE™ + ER-LIVE™ and NucleoLIVE™. Representative images at 48h for vehicle control (0.1% DMSO), AMG900 (aurora kinase inhibitor), CHIR-99021 (Glygocen synthase inhibitor), SU1124 (HGFR inhibitor) and NVP-AEW561 (IFG-1 inhibitor). Green: ChromaLIVE488_Green, Yellow: ChromaLIVE488_Yellow, Orange: ChromaLIVE488_Red, Cyan: ChromaLIVE640, Magenta: CL561 (NucleoLIVE™).
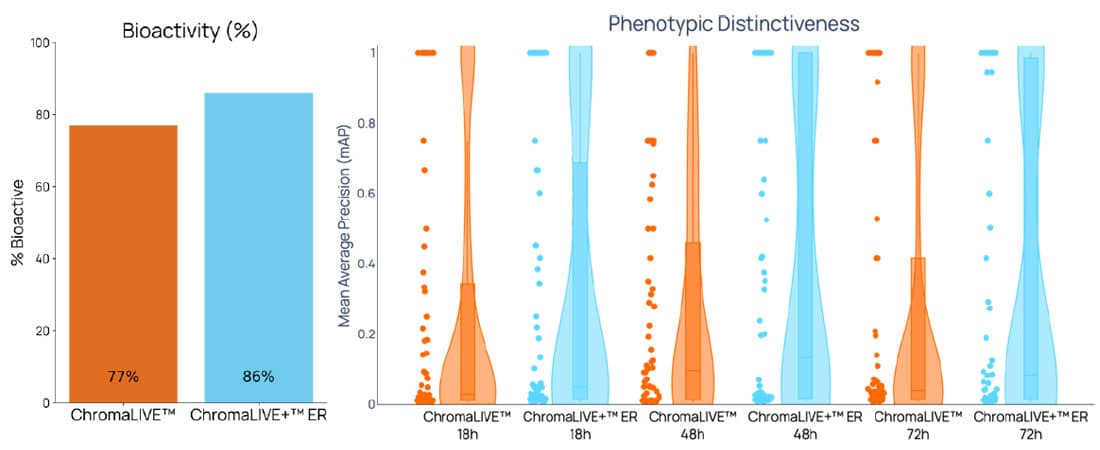
Figure 4. ChromaLIVE™ + ER-LIVE™ improves compound bioactivity detection and phenotypic distinctiveness compared to ChromaLIVE™. U2OS cells were stained with ChromaLIVE™ and Hoechst (100 ng/mL, orange) or ChromaLIVE™ + ER-LIVE™ and NucleoLIVE™ (cyan). A) Morphological profiling with ChromaLIVE™ + ER-LIVE™ and NucleoLIVE™ improved bioactivity detection by 9% for the 90 compounds of the JUMP-MOA plate (average from independent experiments). B) Additionally, ChromaLIVE™ + ER-LIVE™ improved phenotypic distinctiveness (i.e higher distinctiveness indicates more unique morphological signatures between compounds) at several timepoints of the assay.
6. Example Protocol (for kinetic, 2D live-cell assay)
MCF7 cells treated with standard compounds for apoptosis, ER stress and autophagy. MCF7 are cultured in RPMI 1640 complemented with 10% FBS and 1% Penicillin/Streptomycin.
ChromaLIVE™ + ER-LIVE™ and NucleoLIVE™ Dye Dilution and Preparation (Day 0):
- Warm up the ChromaLIVE™, ER-LIVE™ and NucleoLIVE™ dye tubes to room temperature before use and gently spin to collect any dye solution that may remain near the cap
- Dilute 10 µL of ChromaLIVE™, 10 µL of ER-LIVE™ and 10 µL of NucleoLIVE™ in 10 mL culture medium (1000-fold)
- Vortex thoroughly
Cell Culture Protocol with ChromaLIVE™ + ER-LIVE™ (Day 0):
- Harvest and count MCF7 cells
- Resuspend cells in prepared culture medium with ChromaLIVE™ + ER-LIVE™ mix and NucleoLIVE™ at 80,000 cells/mL
- Seed 96-well plate with 100 µL cell suspension per well to a final density of 8,000 cells per well
- Incubate overnight at 37°C, 5% CO2
Standard Compound Preparation and Addition (Day 1):
- Prepare dose-response curves with 10x concentrations, maintaining constant vehicle (0.1% DMSO) solvent concentration
- Prepare negative controls with 0.1% DMSO in complete media
- Distribute 12.5 µL of test compounds or controls per well
Imaging and Data Acquisition (Days 1-3):
- Image 96-well plate at 3h, 6h, 24h and 48h after addition of test compounds
7. Recommended Positive Control Compounds for Cell Death Mechanisms*
Table 4. Doses and treatment durations for MCF7 cells in 2D. Bold represents recommended assay endpoint.
| Cell Death Mechanism | Apoptosis | ER Stress | Autophagy |
|---|---|---|---|
|
Control Compound |
Actinomycin D (1 pM-1 μM)
|
Tunicamycin (10 pM-10 μM)
|
Rapamycin (10 pM-10 μM) 12h, 24h, 48h, 72h |
* Compounds provided as examples only. Validation required for each experimental protocol and cell model.
** Images could be collected more frequently with the appropriate equipment, especially for time-lapse imaging (controlled temperature and CO2, auto-focusing, etc.)